CHD and PAH
CHD and PAH

PAH is a silently progressive disease[1]
PAH, a subgroup of pulmonary hypertension,[2] is a rare, severe and progressive disease characterised by chronic elevation in pulmonary vascular resistance and arterial pressure.*[3] If left untreated, PAH eventually results in right ventricular failure and death.[3][4]
Vascular remodelling in PAH leads to narrowing of the pulmonary arteries and increased pulmonary vascular resistance[3]
Increased pulmonary vascular resistance leads to high right ventricular afterload, hypertrophy, dilatation and, eventually, right heart failure[5]
*The current haemodynamic definition of PAH is mPAP ≥25 mmHg, PAWP ≤15 mmHg and PVR >3 Wood units.[2]
Early identification and intervention is key to changing the course of PAH[6]
PAH is a common complication of CHD[2][7]
CHD-PAH is a common PAH subtype, accounting for 10–20% of cases,[8] and represents a heterogeneous patient population.[9] Patients with CHD-PAH can be classified into one of four main subgroups according to the 2015 ESC/ERS guidelines:[9]
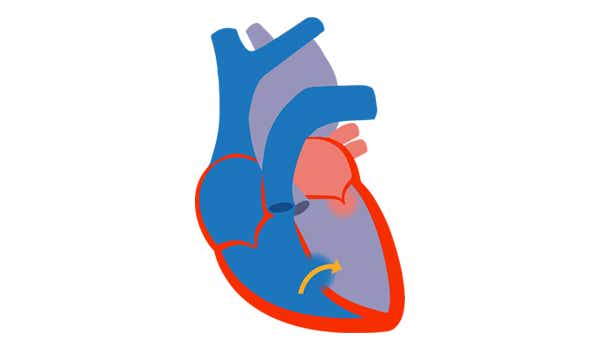
1. Eisenmenger's syndrome[2]
- Includes large defects that develop due to systemic-topulmonary shunts
- Progression to severe elevation of PVR
- Reversed or bidirectional shunting Cyanosis, erythrocytosis and multiple organ involvement
- PAH is present by definition[10]
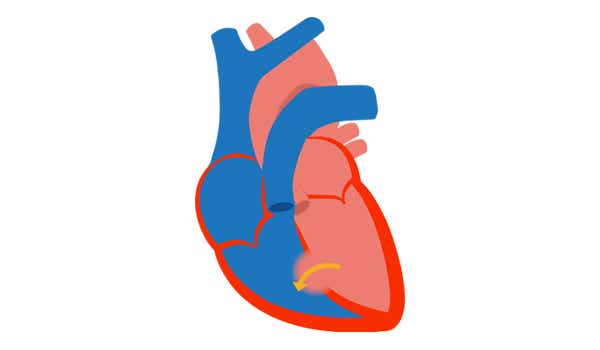
2. PAH associated with prevalent systemic-to-pulmonary shunts[2]
- Includes moderate-to-large defects
- Mild-to-moderate increase in PVR
- Systemic-to-pulmonary shunt is still present
- No cyanosis detectable at rest
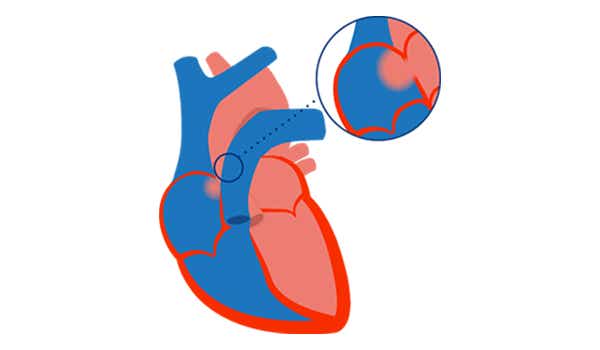
3. PAH with small/coincidental defects[2]
- Small defects (usually ASD <2 cm and VSD <1 cm)
- Marked elevation of PVR
- Clinical presentation similar to idiopathic PAH
- Closing the defects is contraindicated PAH is present by definition[10]
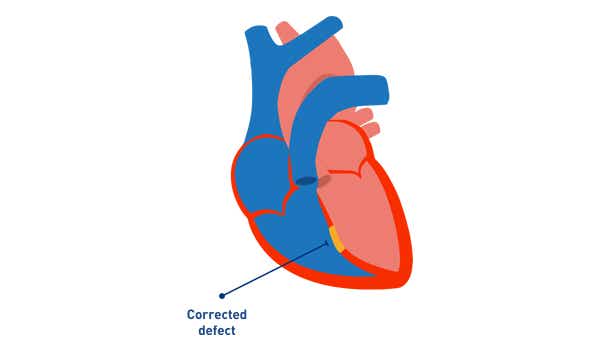
4. PAH after defect correction[2]
- Defect is repaired
- PAH persists immediately after correction or recurs/ develops months or years after defect closure in the absence of significant post-operative haemodynamic lesions
Patients with CHD are at risk of PAH, even when congenital heart defects are corrected[11]
Patients with corrected defects are still at risk of developing PAH[11]

Since PAH can develop over time despite surgery, patients with CHD need regular, long-term screening for PAH after defect correction[10][12]
PAH associated with corrected CHD is increasing in prevalence[13]
Advances in the treatment of CHD over the past few decades have led to an increase in adult CHD survivors, who may then go on to develop PAH following defect correction.[13] The overall prevalence of PAH in patients with corrected simple defects, including ASD and VSD, ranges from 3% to 12%.*[14][7]
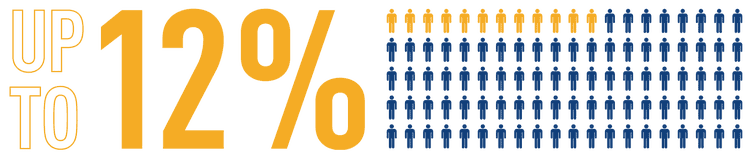
The number of patients who develop PAH after congenital defect correction is increasing[13]
*Data from adult patients with CHD in CONCOR, a Dutch registry (N=2,389),[14] and the Euro Heart survey database (N=1,877).[7]
Patients with corrected CHD-PAH have poor outcomes[15][7]
In patients with corrected CHD, development of PAH is associated with significant worsening in functional limitations and poor long-term survival.*[15][7]
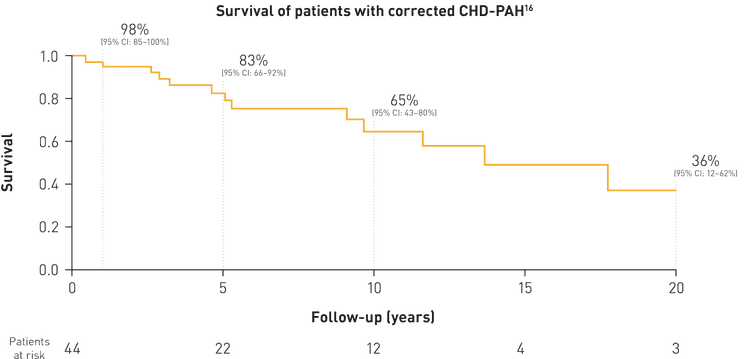
More than 1 in 3 patients with corrected CHD-PAH die within 10 years of PAH diagnosis[15]
*Data from patients with CHD-PAH in an Italian database study (N=192)16 and from patients with CHD in the Euro Heart survey database (N=1,877).[7]
All patients with corrected CHD should be proactively screened for PAH with echocardiography[2][12][16][17][18]
Regular screening of patients with CHD for PAH is recommended in the 2015 ESC/ERS guidelines and the proceedings of the 2018 WSPH.*[2][16] If PAH is suspected, patients should be referred to a specialist PH centre for right heart catheterisation to establish the diagnosis.[2][16][17][18]
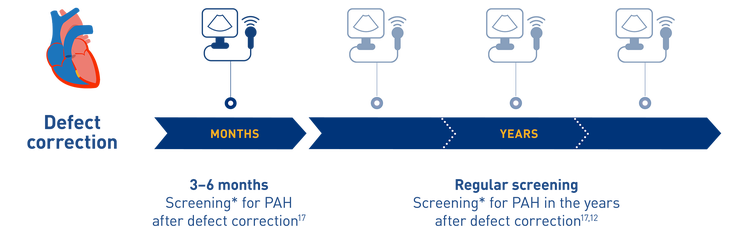
Regular screening can help facilitate early diagnosis and therapeutic intervention for patients with CHD-PAH[12][19]
*Screening should include clinical, echocardiographic and ECG evaluation. Annual screening should be planned for corrected patients who presented with increased baseline pulmonary vascular resistance or with combinations of other predisposing factors.[16]
Screening for PAH in CHD using echocardiography[13][20]
According to the 2015 ESC/ERS PH guidelines, the echocardiographic probability of PAH can be determined based on a number of specific signs;[13] however, these signs may not always apply to patients with CHD due to underlying anatomic and physiological factors.[20]
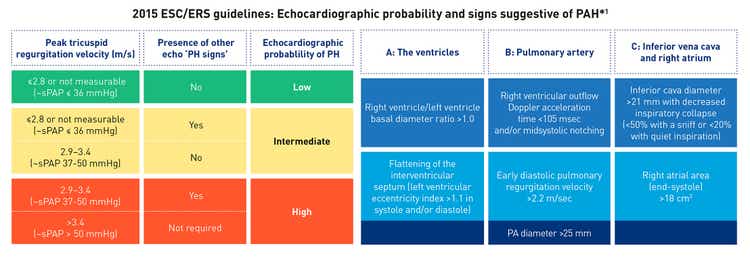
Adapted from Gali. et al. 2016[2]
*Echocardiographic signs from at least two different categories (A/B/C) from the list should be present to alter the level of echocardiographic probability of pulmonary hypertension.[2]
Achieving early identification and treatment of PAH in CHD can be challenging[13][18][20][21]
Patients with CHD can experience a delay of almost 2 years between symptom onset and diagnosis of PAH, which is associated with poor survival outcomes.[19]

Several barriers to early diagnosis and treatment of CHD-PAH exist. Each of them can be overcome by applying the recommendations below:

Patients are often first lost to follow-up during the transition from paediatric to adult services due to a lack of compliance or difficulty adjusting to an autonomous adult healthcare environment[20][22]
In order to be transitioned effectively to adult care, patients should undergo regular follow-up during childhood and adolescence and receive transition support that continues into early adulthood[20][23]
A large proportion of adults with CHD fail to receive regular cardiac care following defect correction; they may be discharged or elect not to seek continued medical advice, and are lost to follow-up[13][20][24]
Regardless of age or success of repair, patients with corrected CHD lost to follow-up should be identified and referred back to specialist care for regular, long-term monitoring[13][20]
Early PAH symptoms can be mild and non-specific, and other cardiopulmonary diseases are often considered before PAH[21]
To help detect PAH, patients with CHD should undergo routine screening for PAH with echocardiography, evaluating peak TRV and the presence of other signs suggestive of PAH[2]

In older patients with CHD, challenging vascular access and reluctance to undergo diagnostic procedures can pose a barrier to assessment of PAH[18]
PAH can be investigated using a combination of non-invasive diagnostic procedures including echocardiography, cardiac MRI and CT scan[18][2]
Identifying PAH as early as possible in patients with CHD, through regular follow-up and screening, is critical to improving their outcomes[19][25][26]
Early treatment of pah can delay disease progression and improve long-term outcomes for your patients with ssc-pah[27][28][29][30]
Find out how your patients could benefit from our current therapies therapies in PAH:

OPSUMIT® is a long-acting, dual ET (endothelin) receptor antagonist (ERA) approved as both monotherapy or in combination for the long-term treatment of pulmonary arterial hypertension (PAH).[31]

UPTRAVI® is an oral selective IP receptor agonist approved as both monotherapy or in combination for the long-term treatment of pulmonary arterial hypertension (PAH).[32]
Help facilitate early diagnosis and timely treatment for your patients with chd-pah and improve their long-term outcomes
✔ PAH is a silently progressive disease[1] and CHD patients are at risk[7]
✔ Patients with CHD may develop PAH even after defect correction[2][11], which is associated with poor long-term survival[15]
✔ Early identification of PAH is critical to improving patient outcomes[19]
- Regular screening for PAH using echocardiography is recommended for all patients with corrected CHD[2][12][16][18]
- For patients with a suspicion of PAH, expedited referral to a specialist PH centre to confirm the diagnosis is advised[16]
Contact
Sales

Product Specialist Pulmonary
Hypertension NL
+31 651 71 19 79
esieber1@its.jnj.com

Product specialist Pulmonary
Hypertension NL
kkimmels@its.jnj.com

Product Specialist Pulmonary
Hypertension NL
+31 611 88 53 92
radriaan@its.jnj.com

Product Specialist Pulmonary
Hypertension NL
+31 625 23 06 83
grietvel@its.jnj.com

PH Specialist
+31 610 96 49 94
lferron@its.jnj.com
Medical

Medical Science Liaison Netherlands
+31 6 25511970
igroot@its.jnj.com
Abbreviations
ACHD, adult congenital heart disease; ASD, atrial septal defect; CHD, congenital heart disease; CI, confidence interval; CT, computerised tomography; ECG, electrocardiography; ERS, European Respiratory Society; ESC, European Society of Cardiology; mPAP, mean pulmonary arterial pressure; MRI, magnetic resonance imaging; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterisation; TRV, tricuspid regurgitation velocity; VSD, ventricular septal defect; WSPH, World Symposium on Pulmonary Hypertension